Technologies to reduce CO2 emissions
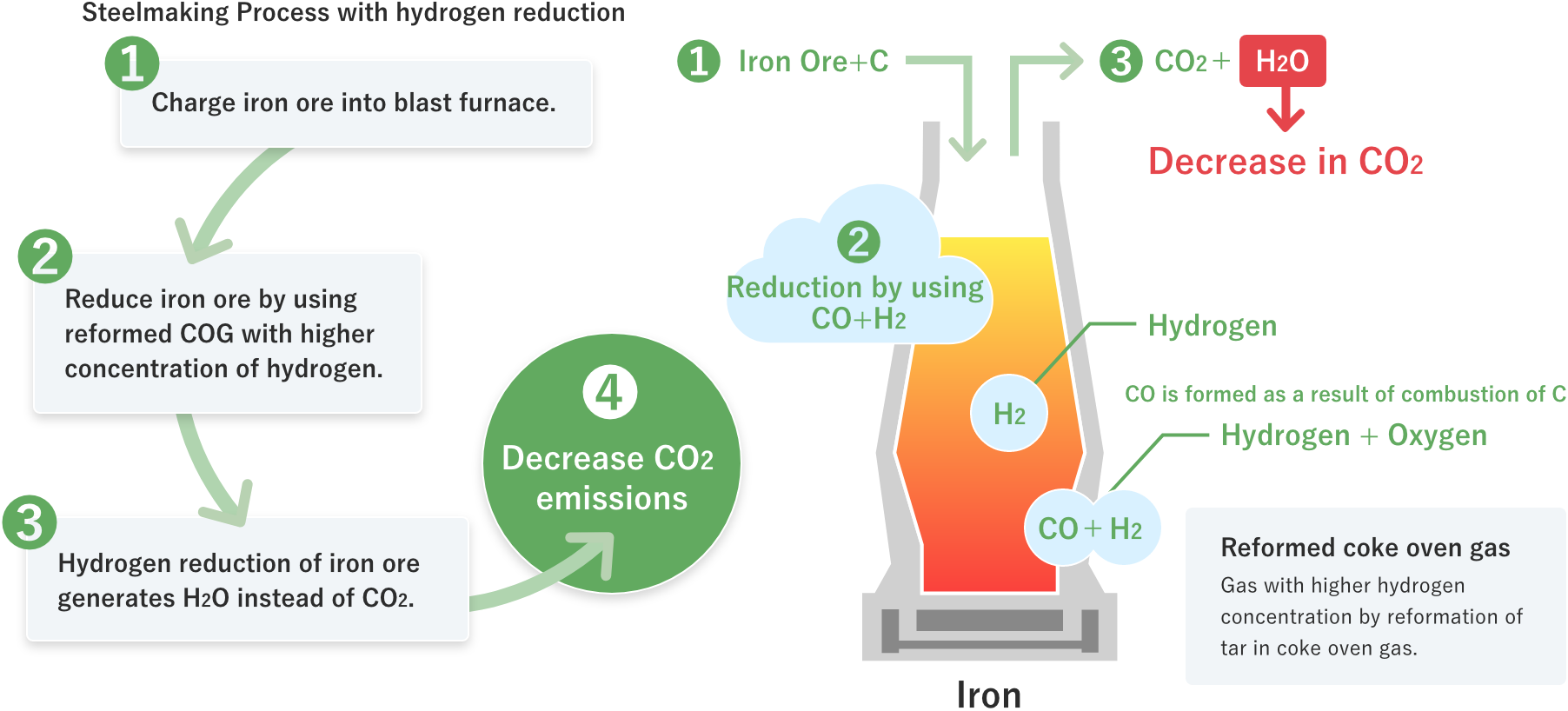
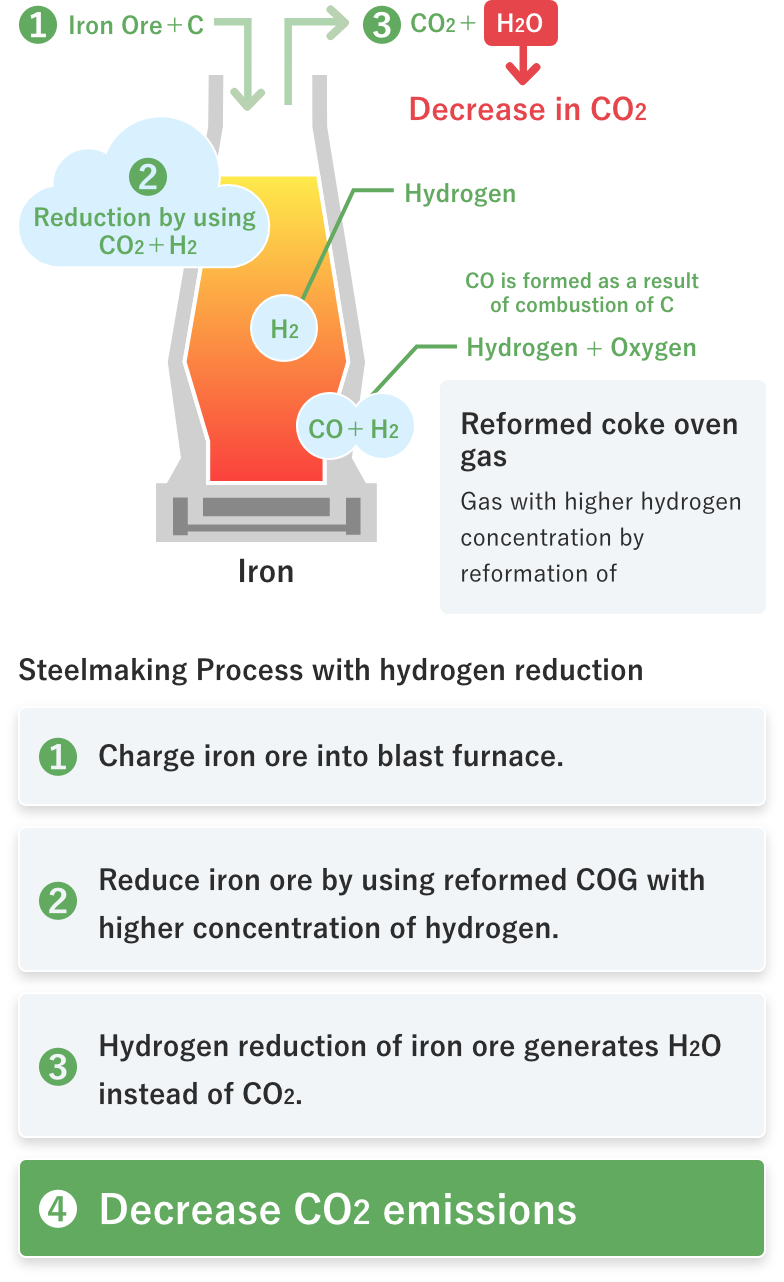
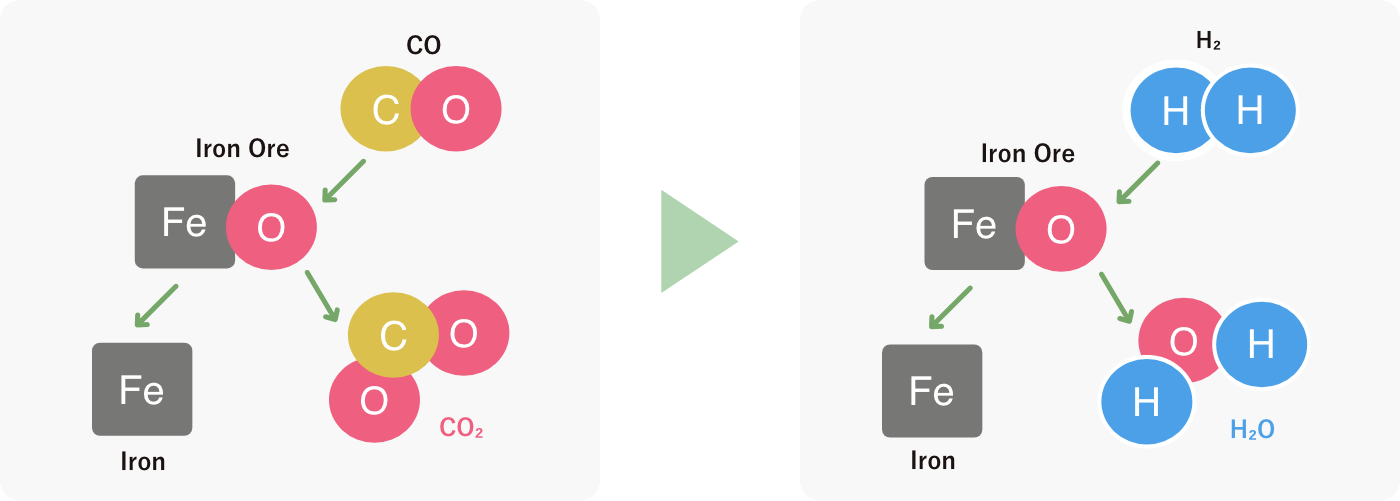
Hydrogen reduction of iron ore generates H2O instead of CO2, leading to decrease in CO2 emissions.
 Interface of chemical reaction
Interface of chemical reaction
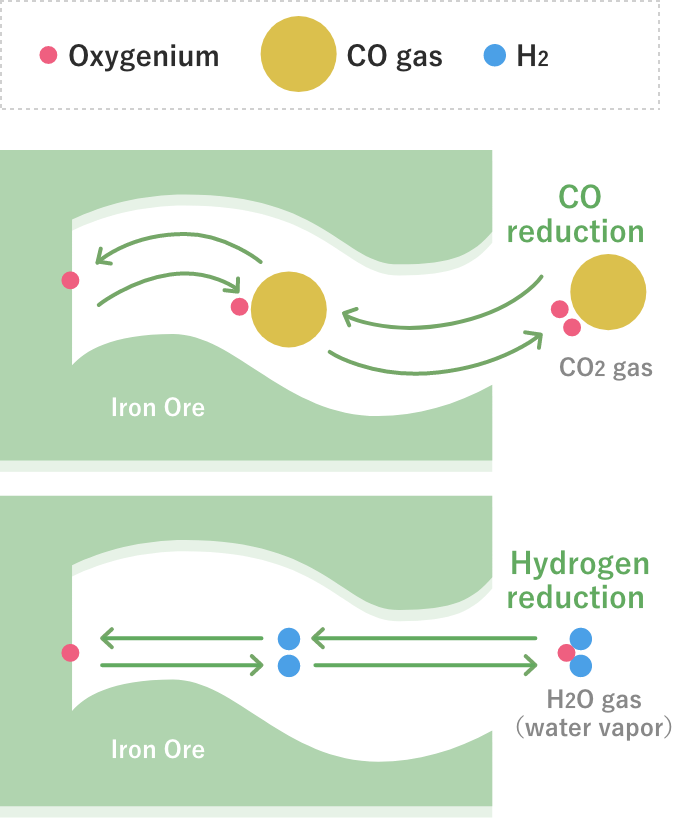
There are three ways of iron oxide reduction (CO indirect reduction, hydrogen indirect reduction, and carbon direct reduction). Of these, carbon direct reduction is a very large endothermic reaction. In a normal blast furnace, the reaction proceeds at a ratio of CO indirect reduction, hydrogen indirect reduction, and carbon direct reduction of about 6: 1: 3. In the long history of blast furnace operations, much effort has been made to reduce the ratio of carbon direct reduction by increasing the ratio of CO indirect reduction, which is an exothermic reaction, but now the operation is already under conditions close to the thermodynamic limit. Therefore, it is difficult to significantly reduce CO2 from the current level.
In COURSE50 project, we are aiming for decreasing of CO2 emissions from a blast furnace by 10% or more by promoting hydrogen indirect reduction, which is an endothermic reaction much smaller than carbon direct reduction, and decreasing the ratio of carbon direct reduction. COURSE50 blast furnace are investigating effects of tuyere injection of COG (coke oven gas) generated in the steelworks, hydrogen gas from the outside, which is expected to be much available in the future, recycled top gas injection that is removed CO2 and H2O, and raw material reactivity on carbon consumption rate.
* You can see the figure enlarged.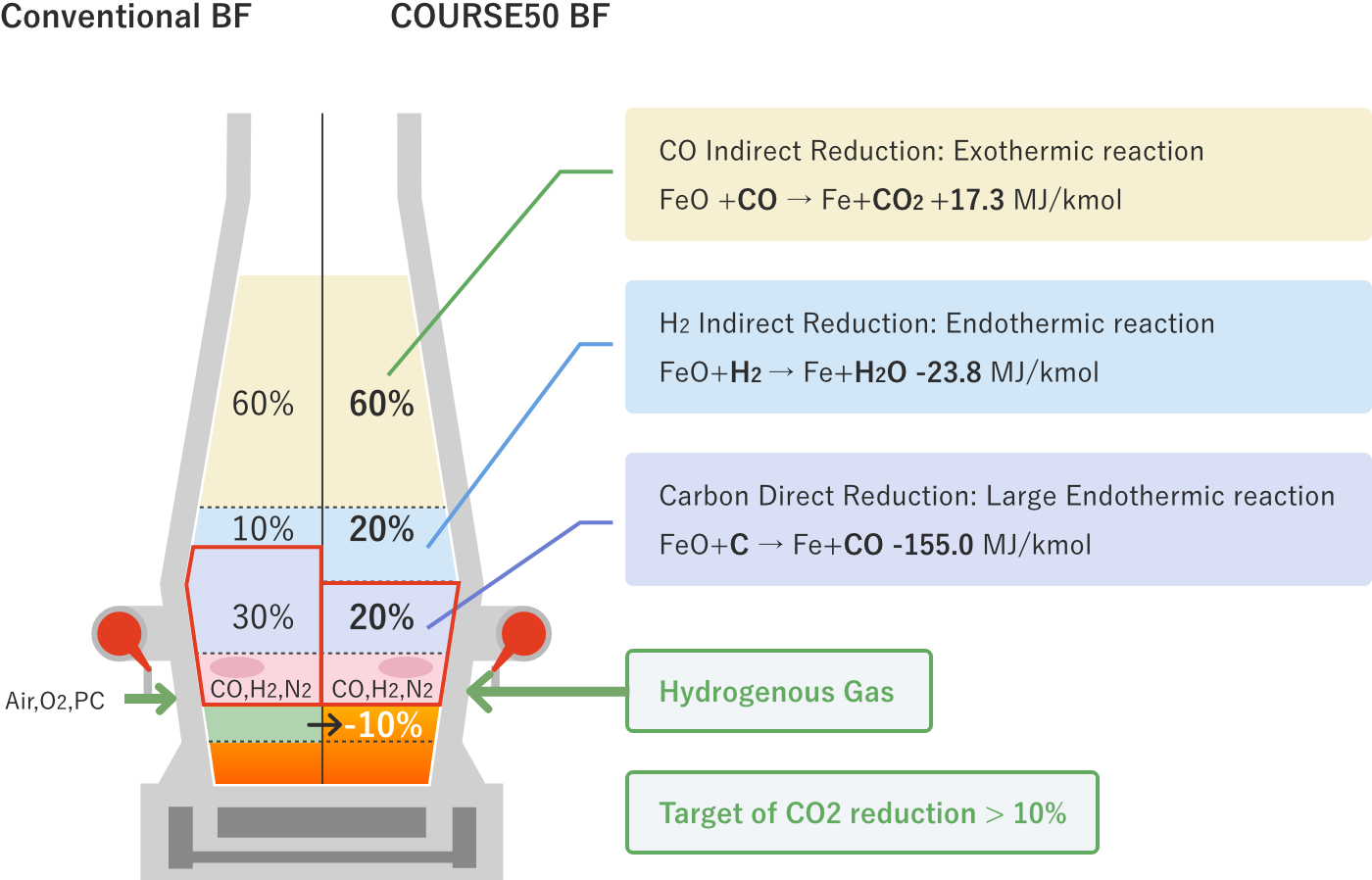
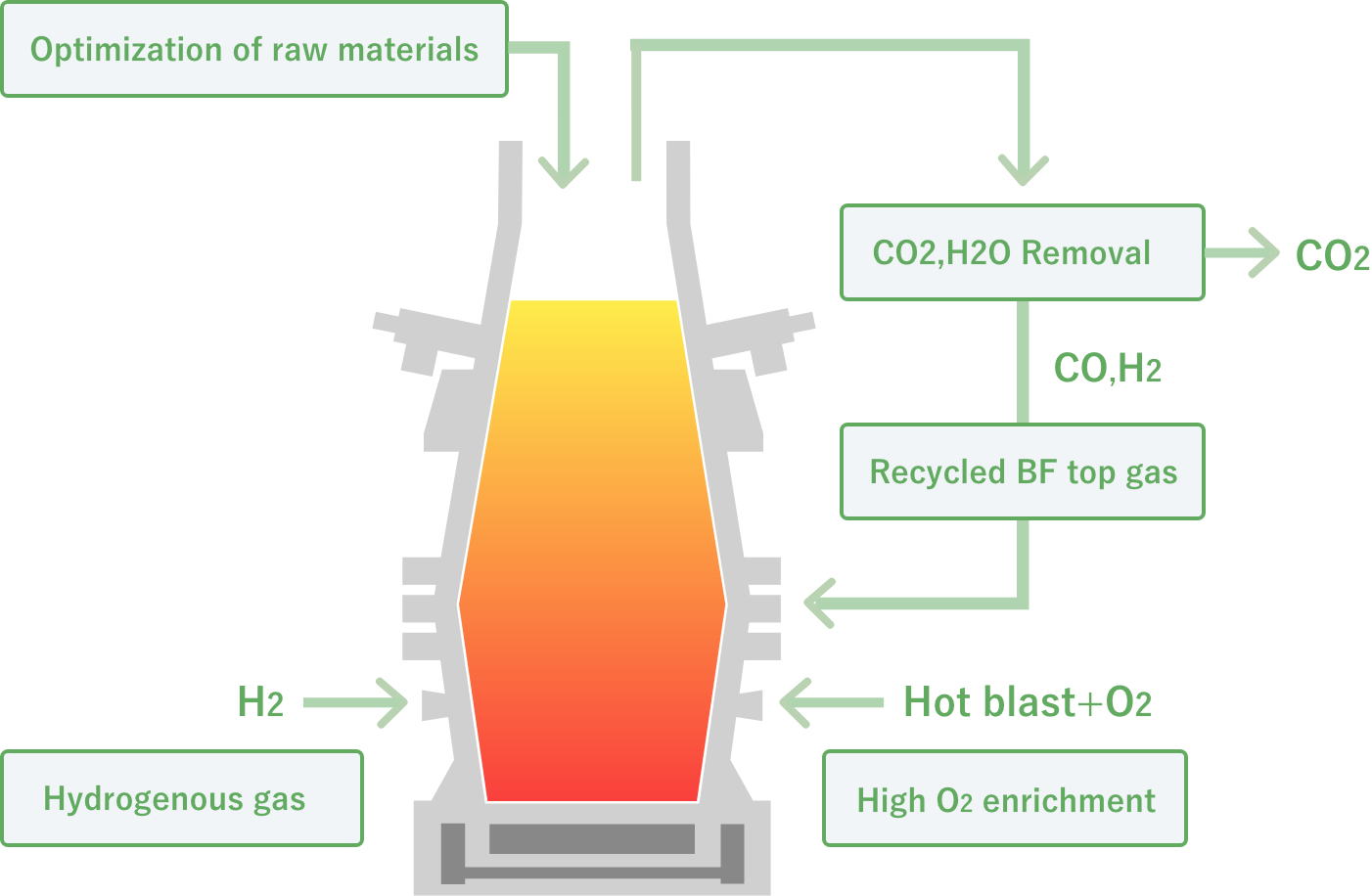
During operation, the mixture of lumpy ore and sinter, which are the raw materials for hot metal, and coke, reductant agent, are charged alternately from above. At the same time, hot blast and oxygen at about 1000ºC, pulverized coal with a diameter of 75 μm or less, and hydrogen containing reductant gas are blown form three tuyeres which are placed at the bottom in order to reduce lumpy ore and sinter. Also, about 4000kg of hot metal and slag (valuables except iron in lumpy ore and sinter) at about 1450ºC are discharged form one tap hole located in the her hearth once every 2 hours. Various data related with her carbon consumption are collected during about 30 days of non-stop operation.



 01
01Hydrogen reduction of iron ore generates H2O instead of CO2, leading to decrease in CO2 emissions.
 02
02Development of chemical absorption technology.
 03
03Development of high-Strength coke production and waste heat recovery technologies for Course 50 process.